

|
Edward Lowton
Editor |


|
| Home> | Efficient Maintenance | >Software | >Software collaboration |
Software collaboration
06 December 2017
Today, many solutions for the pharmaceutical and biotech industry are still not or integrated, requiring more effort to engineer and synchronize data between MES (Manufacturing Execution Systems) and DCS (Distributed Control Systems). Especially in GMP plants (GMP = Good Manufacturing Practices) this additional work has a crucial influence on time to market, quality and profitability. ABB had added a software module from Werum IT Solutions to its Manufacturing Operations Management solution to a
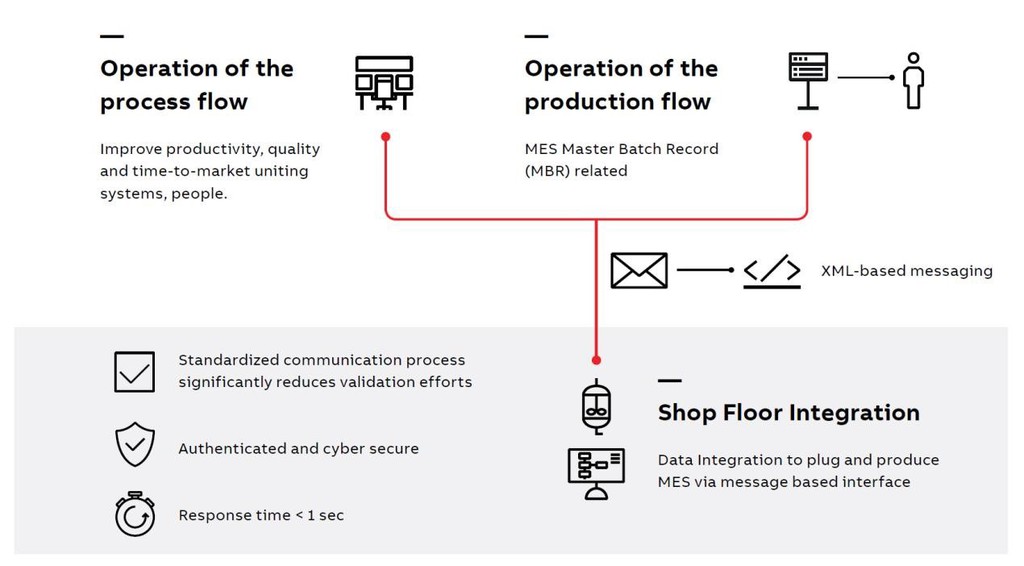
GE Healthcare is among one of the first customers that will implement this Plug & Produce solution. The “Shop Floor Integration for Life Sciences” module is a new addition to the ABB Ability Manufacturing Operations Management (MOM) offering. At their manufacturing site in Uppsala, Sweden, which has one of the world's largest installed capacities for production of chromatographic resins, GE Healthcare will use the new approach to integrate the automation and equipment level for the production of a chromatography medium.
The new solution was presented at Werum IT Solution's annual User Group meeting in Lüneburg, Germany in October 2017. Since 1998, the event has brought together a global network of pharmaceutical and biopharmaceutical manufacturing professionals.
The MES PAS-X from Werum will be integrated with ABB’s flagship Process Control System 800xA: - ABB and Werum have agreed on a long-term roadmap for an aligned offering - Customers will benefit from simplified operation - Faster Master Batch Record (MBR) creation - Joint deployment and validation approach The solution implemented at GE Healthcare will be globally available for sale starting in November 2017 as part of the new Shop Floor Integration Solution for Life Sciences. It provides integration between Manufacturing Execution Systems (MES) and 800xA Batch Management, the integrated Batch system of ABB Ability System 800xA. It includes features for automatic parameter assignment and automatic synchronization of 800xA Batch Management recipe procedures and MES. This automated integration significantly reduces the engineering effort of the integration especially with the high validation requirements in a GMP production environment.
- Variable speed drive eliminates grinder stoppages
- Guaranteed energy savings
- VSDs help hospital cut costs
- Mounting features extend drive applications options
- Joined-up network
- ABB application for pre-emptive maintenance
- Lng tanks rely on abb instrumentation
- Precision boosted
- Smarter tech for safer food
- New head of motors & generators

















